The Development of Scanning Electrochemical Cell Microscopy (SECCM)
- 03 Jan 2020
- Volume 18
- NanoScientific Magazine, Winter 2020
AN INTERVIEW WITH DR. MARTINEDWARDS UNIVERSITY OF UTAH
How w as the SECCM technique developed?
as the SECCM technique developed?
It is said that ‘necessity is the mother of all invention’- the development of scanning electrochemical cell microscopy (SECCM) was no exception. It was developed during my PhD in the group of Prof. Pat Unwin (University of Warwick, UK), out of the desire to answer a scientific question, which was beyond the reach of existing technology. Specifically, we wanted to measure the rates of electrochemical reactions on heterogeneous surfaces, where the heterogeneity was on ~10 nm-1 μm length scale.
In a conventional electrochemical measurement, the entire sample is immersed in solution and the current reports the total rate of electrontransferoccurring over the entire sample. Clearly this is no good if you want to understand the heterogeneity, so we turned to scanned probe microscopy.
At the time, the state-of-the-art in electrochemical scanned probe microscopy involved immersing the entire sample in electrolyte and scanning a microelectrode in close proximity to it. The current at the microelectrode reports the local concentration of electrochemically active species, from which a map of the reactivity of the underlying substrate is inferred.
Scanned microelectrodes had been used to quantify heterogeneity in physical and biological systems ranging from respiration in cell cultures, through electrocatalysis, to corrosion in alloys. However, these techniques had some shortcomings: (1) the current measured depends on both the microelectrodeto-sampledistance and the rate of the electrochemical reaction on the sample surface;to accurately determine the rate alone requires considerable expertise and careful experimental design.(2) Spatial resolution is diminished by diffusion of reaction products, and quantification of rates in low activity regions is obscured by the reaction product from nearby active regions.
SECCM simultaneously overcomes these two challenges. The meniscus at the end of a micro- or nanopipette, when touched to the substrate surface, defines the region in which the electrochemical reaction takes place. There is no diffusion from neighboring regions, as they are not in solution. Moreover, as the current measured comes only from the wetted region, we have a direct measure of the rate of the electrochemical reaction in that region, with no need for complex analysis.
The development of SECCM did not occur in isolation. Fortunately, the Unwin lab (Warwick, UK), where the instrument was developed, already had the physical components from other electrochemical scanned probe microscopes, which were capable of precise positioning and the measurement of small currents. The lab had also previously experimented with electrochemistry in ~100 μm diameter droplets, which was achieved by hand-positioning the meniscus at
the end of a capillary to the surface using manual micro-positioners and a video microscope. SECCM grew from combining these two techniques.
Switching to using an electrochemical signal to detect contact of the meniscus with the surface meant we no longer relied on the video microscope. We could then use much smaller(~100 nm) pipettes, a ~4 orders of magnitude improvement in resolution! Since then, the lateral resolution of the technique has continued to improve, with 10’s nm lateral resolution imaging reported.
To stop the movement of the pipette once the meniscus made contact with the surface and before the pipette would crash into the surface would take super-human reactions, so I programmed an instrument to do it. Fortunately, it
worked out! The instruments today still operate on the same basic principles we developed. However, while the first images ~10 years ago took 2-3 hours and only contained ~400 pixels (20×20), the state-of-the-art - acquiring an image with 10,000+ pixels in only 10 mins – is testament to the hard work of numerous graduate students and postdocs who have continued to refine the technique.
From the humble beginnings of one instrument and a couple of different applications, it has been gratifying tosee the spread of SECCM to other
research groups. The Unwin lab continue to be pioneers in developing instrumentation, theory and applications. However, in a relatively short period, there has been rapid uptake by the community - off the topof my head I can think of at least 15 groups who have adopted the technique, and I’m in no doubt that there are several more.
What are the main advantages of the SECCM technique?
SECCM provides a straight forward way to map the electrochemistry and topography of their samples with nanoscale resolution. The experiments
are not difficult to perform, the probe for SECCM (micro- or nanopipettes) can be cheaply (~$1) and rapidly (~1 min) fabricated from a glass or quartz capillary with very little expertise. Indeed we have had high-school students visit the lab and become competent in their fabrication in only 15 minutes. Interpretation is simple – a higher current represents a higher rate of the electrochemical reaction in the region probed.
The SECCM is a versatile technique. The experimenter is free to choose the chemical system they investigate be altering composition of the solution they fill their pipette with and there is little need for special preparation of samples. Moreover, after the meniscus contacts the surface and defines the electrochemical cell (hence the name of the technique), the experimenter is free to apply the electroanalytical technique that is most appropriate to questions they are asking.
Each time the meniscus touches the sample is the first time that region has seen the electrolyte and can be treated as a new experiment. This makes 10,000 experiments on a single sample a viable possibility, offering exciting possibilities in high-throughput experimentation.
Do you think that SECCM is an important application for researchers and why?
For researchers looking to gain insight into heterogeneity of electrochemical materials, SECCM is a great technique, to which there are very few alternatives. As all electrodes are heterogeneous at some level, the range of systems that it can be applied to is incredibly broad. The measurements made are clear-cut and don’t rely on convoluted analyses. Moreover, when coupled with complementary techniques, one can correlate electrochemical activity with structure, which is the Holy Grail in the field of electrocatalysis.
SECCM allows one to characterize the boundaries between different regions on an electrode surface, which are frequently the location for interesting behaviors. For example, in a recent collaboration with Matt Kanan’s group (Stanford), we recently visualized enhanced CO2 reduction activity at grain boundaries on polycrystalline Au (https://doi.org/10.1126/science.aao3691). Matt had a hunch that this was the case, due to a correlation between the density of boundaries and the activity, but had no direct evidence. SECCM changed this, giving us the opportunity to literally see the current increase over the grain boundaries.
While predicting the future in any field is a fool’s errand, if we take the everexpandingbody of literature as a clue, it seems that the only limitation in the application of SECCM is the creativity of scientists and engineers. Personally, I’m particularly excited to see SECCM push forward understanding in energy technologies, such as batteries and fuel cells, where materials are complex composites.
How was your experience working with Park Systems on developing this technique?
Working with Park Systems has been a collaborative endeavor. The R&D team we work with are a talented team of engineers, but had limited chemical experience. Thus our first task was to deliver a crash course in electrochemistry. Thankfully, they are fast learners!
Park’s experience in both AFM and SICM reassured us that we would be able to perform low mechanical and electrical noise measurements. Fortunately, that the hardware (tip holder & amplifier) for SICM and SECCM are interchangeable, thus the effort in adapting the instruments to perform SECCM has been focused in software. This has involved implementing new control algorithms and ways of presenting the acquired data. Thankfully, through the hard work of all involved, we now perform high quality SECCM imaging on our NX-12 instrument.

Dr. Martin Andrew Edwards
is currently a Research assistant professor at the University of Utah. He completed his PhD under supervisors Prof. P.R. Unwin and Dr. A.L. Whitworth, University of Warwick, Molecular Organisation and Assembly in Cells Doctoral Training Centre (MOAC DTC)–Thesis title: Development of electrochemical probe microscopy and related techniques in 2009. He currently has funding from DURIP - Environmental Electrochemical Scanned Probe Microscope for Investigating Nanoscale Phase Behavior and 3-Phase Boundaries and funding pending at the Department of Energy - Molecular Interrogation of Concerted Electron- and Phase-Transfer Reactions. He has been invited to present at many conferences including "Electrochemistry at three-phase boundaries" ACS Spring meeting; “Resistive Pulse Delivery of Single Nanoparticles to Electrochemical Interfaces” Pittcon, Chicago; “Nanoparticle Dynamics at Electrified Interfaces” Society of Western Analytical Professors (SWAP) Meeting, Salt Lake City;“Single Entity Electrochemistry”, invited seminar, University of Warwick; and “Electrochemistry of Nanobubbles” Utah Energy Symposium, Salt Lake City.
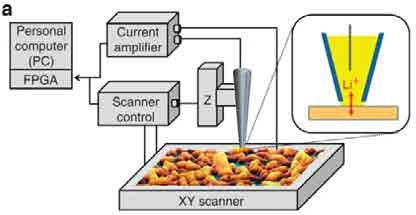

SECCM is a promising technique for measuring the heterogeneity in Li+ insertion into battery cathodes. (above) schematic of SECCM setup
