“Materials Matter” Column 2 - Supramolecular Chemistry, Nanomachines, and AFM -
- 21 Aug 2019
- Volume 17
- NanoScientific Magazine, Summer 2019

Dr. Rigoberto Advincula, Professor,
Macromolecular Science & Engineering Case Western Reserve University
NANOTECHNOLOGY REQUIRES THE USE OF TOOLS NEEDED TO UNDERSTAND PHENOMENA AND MANIPULATE MATERIALS ALL THE WAY TO THE ATOMIC LEVEL. ATOMIC FORCE MICROSCOPY (AFM) IS ONE OF THE MOST IMPORTANT METHODS FOR PROBING AND HARNESSING THE POTENTIAL OF NANOTECHNOLOGY.
The Nobel Prize in Chemistry 2016 was awarded to Sauvage, Stoddart, and Feringa for their work in supramolecular chemistry and it highlighted the need for nanomanipulation and probes to demonstrate stimulation response and motion to molecular and nanomachines. AFM is one of the few methods available to directly visualize and manipulate these nanoobjects by investigating topology and field response in flat surfaces and using specific cantilever-tip to molecule interactions. AFM techniques based on contact and non-contact modes including scanning tunneling microscopy (STM) as well as fieldresponsive methods have enabled quantitative and visualized experiments to correlate with the dynamics of macromolecular and supramolecular chemist.
This column summarizes a webinar on AFM imaging of knotted polymers. This is a summary of the complete webinar given by Dr. Advincula. To view this webinar and other webinars, go to: www.parksystems.com/supramolecular
Can you describe Supremolecular Chemistry?
When we talk about molecules, synthesizing new types of complexes, macromolecular systems that involve metal-to-ligand interaction or even
protein assemblies in enzymes, what is fascinating is that you can use the different types of non-covalent and covalent interactions to create larger molecules with varying complexity or structures that are beautiful and at the same time functional. What opens the door for different types of modular approaches in chemistry is that you can take these building blocks and assemble them in terms of different geometries, taking molecular precursors into different architectures that then can be complex, let’s say, in guest host systems that will allow you to look at binding kinetics or self-assembly towards higher ordered structures. You have a molecular approach to prepare the building blocks that can then result into higher complexity based on the synthesis of these building blocks with finite covalent structures, however, you can prepare different types of non covalent structures or complexation to build larger macromolecules or supramolecular structures that then can have function for recognition, transport and catalysis.
What are Supramolecular Chemistry Molecule Knots?
You can actually have these intricate cyclic structures, polycycles, macrocycles, different types of order where the building of these structures can be achieved through ion complexation, hydrogen bonding, or donor-acceptor systems. The ability to put this together to form larger structures with finite geometry such as a trefoil knot or a Borromean ring or Solomon link has been a fascination of a lot of organic chemistry aficionados, scientists, who would like to be the first to report this type of architecture using these different building blocks. There’s a whole world there of organic chemists who are fascinated with building this supramolecular structures or even trying to observe their formation of extended and higher ordered structures.
What are Synthetic Strategies for Polymer Catenation?
A basic strategy usually involves the use of a more template-directed catenation or binding whereas in the statistical catenation or threading, you don’t have that yield that allows you to isolate a higher yields of specific cyclic structures or catenation whereas by templating it, one can bias the system such that that particular precursor structure can be formed followed by the final step which is in this case ring closure. This basic dichotomy between statistical and templated direction actually is the key towards higher yields or isolation of specific geometries based on molecular bias. That is why templates such as transitional metal, donor-acceptor, hydrogen bonding, or other types of ligand complexation is quite effective in locking or preparing this interlock structure where the final step can be used to close the ring or prepare different types of extended structures such as tubes, calixarene rings, chalice-type of structures, or different geometries that are geometrically feasible.
What are Programmed Knots and Knot Theory?
You can actually program the links to give specific catenated or knotted structures based on the degree of complexation and ligand-to-metal interaction such as it was popularized by Sauvage during the 1990s. You cancreate metal-to-ligand interactions withone metal, two metals, three metals,four metals, and five metals, by closingthe ring, let’s say connecting A and B orX and Y, you will end up actually witha catenated structure of two rings bydemetalation.On the other hand, if you take abidentate ligand and complex twometals within this ligand and close thering, you can actually demetalate that and form the simplest knot or a trefoilknot. What you can see here as you go with an odd and even number of chelation, you can go from a cyclic to a knot, cyclic to a knot, cyclic to a knot, and so on. What’s fascinating here isthat this is a programmable method based on knot theory to create interlinks structures that are defined based on the template that they originated with.
This has to be designed properly or programmed properly in order to get the design cyclic structure or supramolecular molecule. You can simulate or even calculate the type of the degree of interaction to result in that particular geometry. Even higher orders of assembly including proteins, a double helix can result in long range ordering of these structures to result in rods or particle complexes such as micelles, vesicles, and nanoparticles. These are the building blocks to go from molecular to nanomolecular and then mesoscopic. So for example cyclodextrin, cucurbiturils, calixarines are rings that are hydrophobic inside and hydrophilic outside, therefore, making them efficient complexing agents that can render organic molecules more soluble in aqueous or more polar solvents.
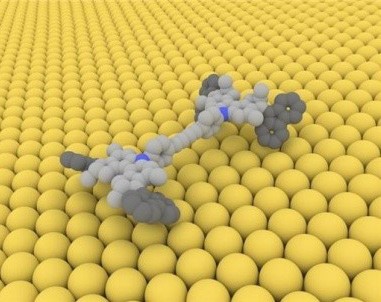
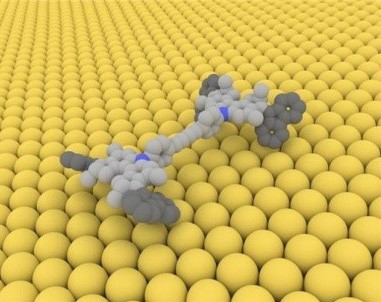
A model of a singlemolecule car that can advance across a copper surface when electronically excited by an STM tip.
Credit: Courtesy of Ben Feringa, co-winner Nobel Prize
Can AFM be used to observe nanomachines or molecular cars?
AFM is a possible tool to actually visualize this supramolecular structure based on the interaction of the cantilever and tip and the assembly or adsorption of the supramolecular structures in flat surfaces. AFM actually plays the key role in observing some of these movement or motion on what we call nanomachines or molecular cars.
Nanocars, when adsorbed on flat surfaces such as mica where the movement of the axle and the rod or wheel and the tire rods show movement over a particular space that can be tracked by atomic force microscopy.
What is the Interest in Polymer Physics about Molecular knots?
The interesting thing is if knots are formed in your DNA during replication, you’re probably be dead. The reason is that cyclic structures or knots are unnatural systems that can mess up your DNA or cause diseases. The fortunate thing is the human body has what we call DNA polymerases that actually correct these structures and destroy them before they can do more harm. In polymer physics, knots and entanglements are a particular interest because chain entanglement as observed with high molecular weight polymers result in changes in the thermo-mechanical, physical, chemical properties of polymers in solution or melt or other real logical parameters or even reptation to tubes or narrow geometries or channels.
Can you explain Knotty Polymers by a Ring-Expansion Strategy
It is possible to make a knotty polymer by ring-expansion strategy. A true knot, the simplest one, the trefoil knot is based on a 3-1 structure. The question is how can we prepare this and can we observe this indirectly and directly. Synthetically, we just extended the catenated structures into this bidentate ligand and the bi functional ligand was then closed and then finally, the caprolactone was polymerized form a polycaprolactone system. Of course, demetalation when unfolded should give a trefoil knot.
Just to confirm that we can observe this by UV-Vis spectroscopy, NMR, or even x-ray photoelectron spectroscopy to confirm the presence of copper and tin on the molecules showed that we have a good correspondence with the drone structures and the empirical proof.
GPC was used to look at the changes in the hydrodynamic volumes. Let me emphasize that a ring structure compared to a linear polymer will have a lower hydrodynamic volume even though they have the same molecular weight. For example, here in this GPC trace, you will see a linear polymer equivalent of a knotted polymer of the same molecular weight but the knotted polymer has a higher retention volume indicating that even though the molecular weight is the same, the hydrodynamic volume of the knotted macromolecule is lower or smaller. The proof is on the AFM. Actually, this was the first report of a trefoil knot synthetic polycaprolactone that was observed on mica and then upon closer examination of that adsorbed macromolecule indeed the trefoil knot structure was confirmed.