Magnetic Nanoscavengers: The New Trend To Improve Water Quality
- 31 Jan 2021
- Volume 21
- NANOscientific Magazine, Winter 2021
Estephany Santiagoa , Georgina Pina-Luisa, Marisela Martinez-Quirozb , Oscar PerezLanderosc , Navor Rosas-Gonzálezc , Benjamin Valdez-Salasc, Mercedes T. Oropeza-Guzmána
Introduction
Reusing water is mandatory in regions where water scarcity is due to climatic conditions, but also it offers the opportunity to mitigate water pollution by reducing the discharge of wastewater to natural surface waters.[1,2] The challenge is to develop sustainable processes to recover clean water from wastewater avoiding the use of non-degradable materials (synthetic polymers and resins), keeping low energy consumption and diminishing the investment cost to operate the technology. Water conditioning technologies such as inverse osmosis, chemical precipitation, ion exchange, and electrochemical removal, are generally used to improve water quality and aesthetics (such as alkalinity and hardness) after primary and secondary processes.[3,4] However, all of them are onerous and require meticulous maintenance to keep efficient operations and improve water quality.[3,5,6] In this pathway, we propose for the first time, the use of magnetic nanoscavengers with high potential to remove hardness and alkalinity from reclaimed water (RW). With this innovation we look to increase the water potential reuse in a simple magnetic contact stage to trap salts. In this paper three types of eco-friendly magnetic nanoparticles were prepared using chitosan, nanodiamond powder, and grafted chitosan. All of them were coupled with magnetite nanoparticles to obtain magnetic chitosan (MCH), magnetic nanodiamond (MND), and magnetic carbamoyl chitosan (MCCH). All the materials were characterized, previous their use, by FT-IR, STEM, Zeta Potential, DLS, and TGA studies, proving the binding between magnetite and the modifiers. Furthermore, RW in contact with magnetic nanoscavengers was used as a model to evaluate their alkalinity and hardness removal capacity. We found out that each magnetic nanoscavenger proved to be effective for trapping and removing alkaline carbonates from reclaimed water, and we demonstrated it by different techniques. At the same time, we have the possibility of recovering them for further use, reducing the operation cost. Resulting residues are not at all toxics and do not require special handling. With all these characteristics we can classify the magnetic nanoscavengers as sustainable nanomaterials.
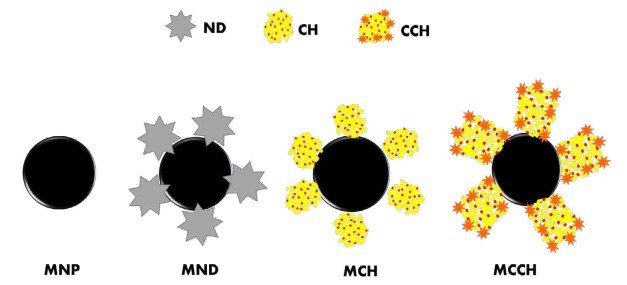
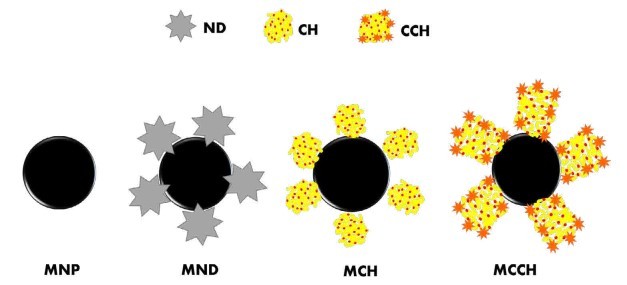
Figure 1. Schematic illustration of the formation of the nanoscavengers MND, MCH, and MCCH
Experimental
Low molecular weight chitosan and nanodiamond powder were used as obtained from the suppliers, Carbamoyl Chitosan was prepared with the methodology reported by Martinez-Quiroz et al. [7] Magnetic nanoparticles and Magnetic nanoscavengers (MCH, MND, MCCH) were synthesized via chemical co-precipitation under alkaline condition inspired in the methodology reported by Liu et al[8] with slight modifications. All the materials were characterized by SEM/ STEM (Tescan Lyra 3GM), FT-IR (Shimadzu FT-IR Spirit ATR mode), TGA (Perkin Elmer equipment TGA 4000, DLS and Z potential (Anton Paar Litesizer 500) RW samples were taken from the wastewater treatment plant “La Morita” (Tijuana, México). With those samples we prove magnetic nanoscavengers effectiveness to remove alkalinity and hardness. The RW was treated with the four magnetic nanoscavenger to study the improvement of the water quality in terms of alkalinity and hardness removal. This study was performed at pH 7, 8, and 9. A dosage of 30 mg per liter of water of magnetic nanoscavenger (MNP, MND, MCH, MCCH) was weighed and then placed into the RW sample at the pH previously adjusted. The solution was sonicated for 5 minutes and then placed into an orbital shaker for 10 minutes at 360 rpm. After this time, the nanoparticles were recovered by magnetic decantation. Alkalinity and hardness were measured with standard methods for that purpose.
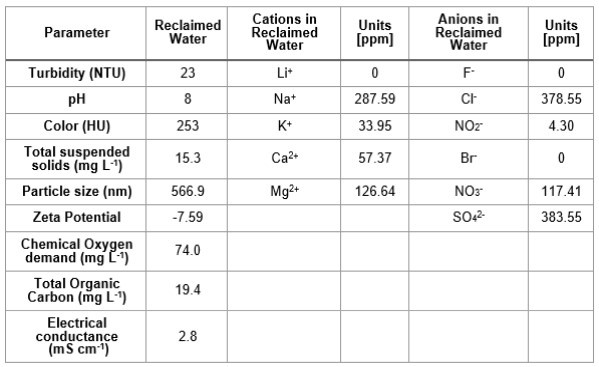
Table I. Composition of reclaimed water obtained from the wastewater plant treatment "La Morita" in Tijuana, Baja California, México.
Results and discussion
RW original characteristics are shown in table I. Special attention is posed on alkalinity and hardness, since these parameters are among those that need to be improved to increase water potential reuse. The first strategy was done measuring the Zeta potential of raw RW, as well as the hydrodynamic diameter of residual suspended particles, for further comparison with Zeta potential of magnetic nanoscavengers dispersions. Figure 1 illustrates the nanometric conception of magnetic nanoparticle with the three different types of nanostructures. These molecular assemblies were obtained by the coprecipitation method and its stability was tested by several techniques. Figure 2. shows the physical and chemical properties of magnetic nanoscavengers by FT-IR, TGA, Zeta Potential, DLS and STEM, proving the suitable binding between magnetite and the modifiers. Among the magnetic nanoscavengers (MND, MCH and MCCH) the surface chemistry can be related to its physical aspect. For example, particle size vs. Zeta potential suggests that MCCH is composed of very thin particles that has an acid-base performance. Later on SEM images show the low agglomeration level due to the carbamoyl benzoic acid incorporated to the magnetic nanoparticle. In contrast MCH shows larger particles and also a completely opposite DLS profile. Figure 3a. corresponds to the Zeta potential and DLS practiced to RW, showing low Zeta potential values at pH<4 and negative values reaching -14 mV at pH 10. This result is certainly due to water content. In contrast DLS shows larger particles at greater pH suggesting some salts precipitation when diminishing proton concentration. In Figure 3b a bar graph shows the alkalinity and hardness of RW at three different pH values that will serve to estimate the percentage of alkalinity and hardness removal when magnetic nanoscavenger are in direct contact with RW. Figure 4. allow to evaluate the action of magnetic nanoscavengers by the hydrodynamic diameter and Zeta potential. These techniques are commonly used to characterize colloidal nanoparticles; and in this study both of them demonstrated to be important tools to evaluate water quality before and after a conditioning process. Figure 5. corresponds to the final result of alkalinity and hardness removal at three different pH for the four magnetic nanoscavengers. Concerning MCH and MCCH they showed to be the most performant for hardness removal. In the case of alkalinity MCH and MCCH are doing the same effect at pH 9. MCH showed to be the more performant nanoscavenger, in particular at pH 8, that is the closest value to the raw RW.
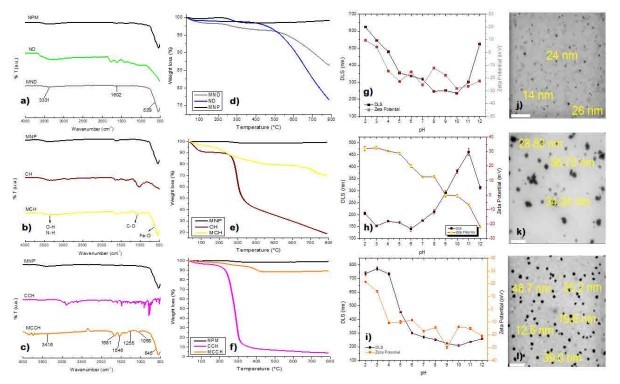
Figure 2. Characterization of Magnetic nanoscavengers by: FT-IR: a) MND, b) MCH c) MCCH; TGA: d) MND, e) MCH, f) MCCH; DLS/Zeta Potential vs pH: g) MND, h) MCH, i) MCCH; and STEM: j) MND, k) MCH, l) MCCH. The white line represents a scale bar of 200 nm.
Conclusions
Thanks to the chemical modification of magnetite, it was possible to obtain three different magnetic nanomaterials (MND, MCH, and MCCH) which act as magnetic nano-softeners. We found out that each magnetic nanoscavenger proved to be effective for trapping and removing carbonates from reclaimed water, and we demonstrated it by different techniques. At the same time, we have the possibility of recovering magnetic nanoscavengers for further use and reduce the operation cost. With all these characteristics we can classify the prepared materials as sustainable nanomaterials.
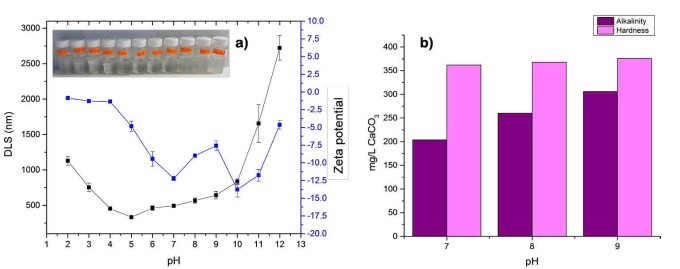
Figure 3. a) DLS (black line) and Zeta Potential (blue line) of the RW from the WWTP “la Morita”. The pH was adjusted using solutions of 0.001 M HCl and 0.001 M of NaOH. b) Alkalinity(purple) and hardness (pink) measurements for RW from the WWTP “La Morita” at pH 7, 8, and 9 expressed as mg L-1 of CO3.
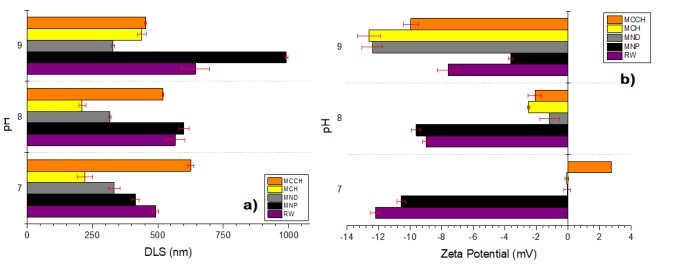
Figure 4. DLS measurements of untreated RW (purple), and RW treated with MNP (black), MND (gray), MCH (yellow), and MCCH (orange) at pH of 7, 8, and 9. d) Comparison of the Zeta Potential measurements of untreated RW (purple), and treated with MNP (black), MND (gray), MCH (yellow), and MCCH (orange) at pH of 7, 8, and 9
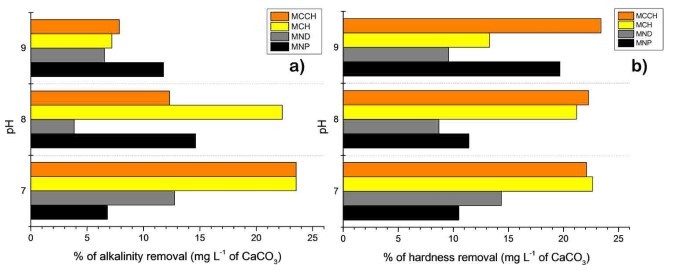
Figure 5. a) Comparison of the alkalinity removal of MNP (black), MND (gray), MCH (yellow), and MCCH (orange). b) Comparison of the hardness removal of MNP (black), MND (gray), MCH (yellow), and MCCH (orange). At pH of 7, 8, and 9, expressed as percentage in mg L-1 of CaCO3.
References
[1] Escalante Estrada, V. E. Reúso Del Agua Residual En México, Curso inte.; Instituto Mexicano de Tecnología del Agua: Morelos: Morelos, México, 2004.
[2] THE WORLD BANK. Water Resources and Environment Technical Note F.3; DAVIS, R., HIRJI, R., Eds.; The International Bank for Reconstruction and Development/THE WORLD BANK, 2003.
[3] Crittenden, J. C.; Trussell, R. R.; Hand, D. W.; Howe, K. J.; Tchobanoglous, G. MWH’s Water Treatment: Principles and Design; John Wiley & Sons, 2012.
[4] Fu, F.; Wang, Q. Removal of Heavy Metal Ions from Wastewaters: A Review. J. Environ. Manage. 2011, 92 (3), 407–418.
[5] Rajasulochana, P.; Preethy, V. Comparison on Efficiency of Various Techniques in Treatment of Waste and Sewage Water – A Comprehensive Review. Resour. Technol. 2016, 2 (4), 175–184.
[6] Mahamuni, N. N.; Adewuyi, Y. G. Advanced Oxidation Processes (AOPs) Involving Ultrasound for Waste Water Treatment: A Review with Emphasis on Cost Estimation. Ultrason. Sonochem. 2010, 17 (6), 990–1003.
[7] Martínez-Quiroz, M.; López-Maldonado, E. A.; Ochoa-Terán, A.; Pina-Luis, G. E.; Oropeza-Guzman, M. T. Modification of Chitosan with Carbamoyl Benzoic Acids for Testing Its Coagulant-Flocculant and Binding Capacities in Removal of Metallic Ions Typically Contained in Plating Wastewater. Chem. Eng. J. 2018, 332, 749–756.
[8] Liu, X.; Ma, Z.; Xing, J.; Liu, H. Preparation and Characterization of Amino–Silane Modified Superparamagnetic Silica Nanospheres. J. Magn. Magn. Mater. 2004, 270 (1), 1–6.