Chemical Imaging Is Leading To Better Molecular Information From The Micro To Nanoscale
- 30 Sep 2020
- Volume 20
- NANOscientific Magazine, Summer 2020
Kevin Yeh1 , Seth Kenkel1 , and Rohit Bhargava1,2,3
1 Beckman Institute for Advanced Science and Technology
2 Departments of Bioengineering, Mechanical Science and Engineering, Electrical and Computer Engineering, Chemical and Biomolecular Engineering, and Chemistry
3 Cancer Center at Illinois, University of Illinois at Urbana-Champaign, Urbana, Illinois, United States
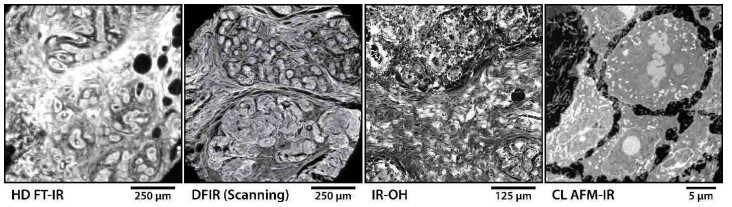
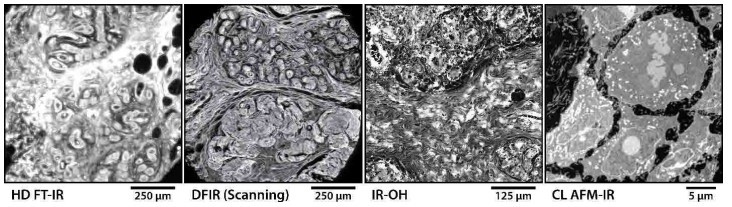
Figure 1. Representative data acquired by CI microscopy systems including: a transmission HD FT-IR system imaging a breast tissue core shown at the Amide I band; a transflection DFIR direct point scanning system also imaging breast tissue when tuned to Amide I; an IR-OH system imaging the PT effect with WF illumination on the Amide II band; and lastly, an AFM-IR system with CL controls imaging at Amide II the protein distribution of subcellular structures within a single cell.
Vibrational infrared (IR) spectroscopy on a spatially resolved microscopic scale, a mainstay of the emerging field of chemical imaging (CI), has seen unprecedented growth in recent years due to the diversity of new instrument designs and measurement techniques. CI expands on our knowledge of traditional microscopy by deriving its contrast intrinsically from the molecular composition of the sample. Across research ranging from medicine to material science, CI has the potential to enable many impactful contributions. For instance, by introducing automatic quantitative processes to pathology or analysis of microplastics via improvements, CI instrumentation are positioned to take advantage of recent revolutions in data science.
Until recently, there have been few realistic options to traditional FT-IR imaging. While modern FT-IR microscopes are an excellent research analytical tool, unsurpassed especially when prior knowledge of the sample is unavailable, its limitations are apparent for highthroughput routine inspection where we can safely assume a priori information, or for applications where sub-diffraction spatial resolution is necessary. These limitations largely were a result of the low light intensity from globar sources, simply a heated silicon-carbide rod, which was the only option practical for IR imaging. Advances toward higher definition (HD) FTIR resulted from improved theoretical [1] understanding, but it was not until recent years with broad commercial availability of new quantum cascade lasers (QCL) that turnkey solutions provided a clear path forward. These high-intensity mid-IR sources were combined as multi-laser assemblies that together were tunable across the entire mid-IR fingerprint region. Their narrow-band emission, in conjunction with their discrete single wavelength broad tunability, allowed systems to measure only the precise spectral features of interest, thus enabling high-throughput, discrete frequency infrared (DFIR) spectroscopy.
For targeted applications, the number of discrete spectral bands required for analytics, especially with the recent developments in data science and machine learning (ML), can often be reduced to sufficiently few with limited loss of accuracy. It is often faster to acquire discrete bands sequentially, contrary to the advantage of simultaneous measurement of a wide spectral range as required by a Fourier transform. The most influential spectral features are chosen through careful design, often requiring significant domain expertise, as is the case with most traditional ML techniques. The process of feature extraction and metric definition is often based on prior scientific knowledge and there is a limit on the number of degrees of freedom that can realistically be defined. Thus, by being reliant on the developer, the performance of these older learning algorithms is capped. New deep learning networks require very little human intervention by comparison. Their performance scales by amount of data and computational power. Especially for biomedical applications, these advances in data science coupled with new CI technology for acquiring vast amounts of information rapidly with cheap computational power and storage, now have the potential to revolutionize diagnostic histopathology and translate CI toward practical clinical use.
The earliest DFIR imaging systems simply retrofitted FT-IR microscopes with a QCL [2, 3]. Over the next several years, several research groups and companies designed new microscopes specifically for integration with QCL sources that specifically take advantage of their many unique properties. The first type of DFIR microscope relies on the direct measurement of residual infrared light after absorption by the sample. These DFIR microscopes have demonstrated drastic improvements in resolution, signal-tonoise ratio (SNR), and imaging speed, thus for the first time, putting CI digital pathology within realistic reach of clinical translation. Systems designed around widefield (WF) laser illumination and utilizing the multi-channel advantage of arrayed detectors have shown exceptional imaging speeds [4]. Meanwhile, scanning systems that tightly focus the coherent laser light into a single spot only a few microns wide [5] and sequentially mapping the sample with high-speed stages, have demonstrated full-slide imaging with unsurpassed spatial and spectral quality at IR diffraction limited resolution [6]. The flexibility of such a platform has also led to expansion of features including simultaneous acquisition of multiple DFs [7] as well as the ability to discern molecular orientations through the measurement of linear dichroism [8].
Recent progress in photothermal (PT) detection provides new opportunities for high resolution CI not possible with conventional IR microscopy. Instead of recording attenuation of IR light, PT instruments encode molecular absorption indirectly by measuring the photoinduced, thermal mechanical response of the sample. Visible Microscopy (VM) and Atomic Force Microscopy (AFM) are the two most common detection methods, both offering resolution beyond the IR diffraction limit. Most notably, VM has shown potential for integrating CI into modern optical microscopy prevalent in clinical and research workflows for histopathology, an IR-optical hybrid (IR-OH) approach [9], achieving resolution at the sub-micron scale. Similarly, when coupled with AFM scanners, PT detection extends CI to the nanoscale, enabling the study of subcellular molecular chemical structures at high resolution for the first time. Despite the apparent benefits in resolution, understanding of the processes involved in image formation is limited, resulting in uncertainty in the recorded data. Consequently, these techniques have been reserved for experts whose experience is vital for designing experiments with tightly controlled conditions to mitigate artifacts. Although this approach has seen some success, the applications are obviously limited. Improving PT instruments guided by understanding of image formation offers an alternative to this approach. For example, theory-driven design has led to recent advancements in AFMbased IR (AFM-IR) spectrosocpic imaging instruments. To first approximation, the deflection signal of conventional AFM-IR is proportional to the IR absorption near the probe tip; however, the AFM cantilever introduces additional signal correlated to local mechanical properties of the sample. Guided by rigorous analytical modeling of the cantilever, better AFM-IR instruments have been designed incorporating additional measurements [10] and advanced controls [11] to not only correct this effect but also improve the noise of the recorded signal by a factor of five. Each innovation enables new possibilities for CI at the nanoscale such as accurate compositional mapping of biological samples [12]; however more theoretical progress is required to further the limits of detection. Although PT detection is useful for measuring nanoscale molecular information, understanding the image formation is essential for navigating the complex physics governing the recorded signal.
With the extent of progress across theoretical understanding, instrument design, and data analytics, CI is heading to better data for a multitude of new applications, from the microscale to the nanoscale, that have not been previously feasible. Representative data from state-ofthe-art CI microscopy systems, as shown in Figure 1, demonstrate a new scope of biomedical experiments addressable by these modern capabilities. Nevertheless, each of these systems offer unique potential. Systems based on measurement of IR light will be faster simply due to the lack of measurement overhead where each essentially instantaneous detector measurement, sometimes from a single laser shot, can directly map to a single pixel on the image. However, due to the longer wavelength of IR light, these systems are also IR diffraction limited at best and may not sufficiently resolve fine morphologic features. On the other hand, the techniques that rely on a photo-induced sample response can offer substantially higher resolution, in many cases, depending on the resolution limit of the probe instead of the IR beam. Since substantially higher laser power is required to generate a measurable deformation, this restricts possible area of IR illumination typically requiring focused single-point excitation, while also often relying on lock-in amplification or interferometry. Therefore, these techniques are much slower due to the quantity of raw measurements required and its subsequent computation in order to generate each data point. It is important to understand the properties and tradeoffs of these techniques and to choose appropriately based on the intended application. The diversity of capabilities provided by this new generation of CI microscopy systems presents scientists with tools appropriate for extracting new information from samples for a broad range of investigations at all length scales.
(1) Reddy, R. R. K. R.; Walsh, M. M. J. M.; Schulmerich, M. V; Carney, P. S.; Bhargava, R. High-Definition Infrared Spectroscopic Imaging. Appl. Spectrosc. 2013, 67 (1), 93–105.
(2) Kole, M. R.; Reddy, R. K.; Schulmerich, M. V; Gelber, M. K.; Bhargava, R. Discrete Frequency Infrared Microspectroscopy and Imaging with a Tunable Quantum Cascade Laser. Anal. Chem. 2012, 84 (23), 10366–10372.
(3) Yeh, K.; Schulmerich, M.; Bhargava, R. Mid-Infrared Microspectroscopic Imaging with a Quantum Cascade Laser. In SPIE; Druy, M. A., Crocombe, R. A., Eds.; 2013; Vol. 8726, p 87260E.
(4) Yeh, K.; Kenkel, S.; Liu, J.-N. J.-N.; Bhargava, R. Fast Infrared Chemical Imaging with a Quantum Cascade Laser. Anal. Chem. 2015, 87 (1), 485–493.
(5) Yeh, K.; Bhargava, R. Discrete Frequency Infrared Imaging Using Quantum Cascade Lasers for Biological Tissue Analysis. In SPIE; Mahadevan-Jansen, A., Petrich, W., Eds.; 2016; Vol. 9074, p 970406.
(6) Mittal, S.; Yeh, K.; Suzanne Leslie, L.; Kenkel, S.; Kajdacsy-Balla, A.; Bhargava, R.; Leslie, L. S.; Kenkel, S.; Kajdacsy-Balla, A.; Bhargava, R. Simultaneous Cancer and Tumor Microenvironment Subtyping Using Confocal Infrared Microscopy for All-Digital Molecular Histopathology. Proc. Natl. Acad. Sci. 2018, 115 (25), E5651–E5660.
(7) Yeh, K.; Lee, D.; Bhargava, R. Multicolor Discrete Frequency Infrared Spectroscopic Imaging. Anal. Chem. 2019, 91 (3), 2177–2185.
(8) Phal, Y.; Yeh, K. L.; Bhargava, R. Polarimetric Infrared Spectroscopic Imaging Using Quantum Cascade Lasers. In Advanced Chemical Microscopy for Life Science and Translational Medicine; Simpson, G. J., Cheng, J.-X., Min, W., Eds.; SPIE, 2020; Vol. 1125210, p 34.
(9) Schnell, M.; Mittal, S.; Falahkheirkhah, K.; Mittal, A.; Yeh, K.; Kenkel, S.; Kajdacsy-Balla, A.; Carney, P. S.; Bhargava, R. All-Digital Histopathology by Infrared-Optical Hybrid Microscopy. Proc. Natl. Acad. Sci. 2020, 117 (7), 3388–3396.
(10) Kenkel, S.; Mittal, A.; Mittal, S.; Bhargava, R. Probe–Sample Interaction-Independent Atomic Force Microscopy–Infrared Spectroscopy: Toward Robust Nanoscale Compositional Mapping. Anal. Chem. 2018, 90 (15), 8845–8855.
(11) Kenkel, S.; Mittal, S.; Bhargava, R. Closed-Loop Atomic Force Microscopy-Infrared Spectroscopic Imaging for Nanoscale Molecular Characterization. Nat. Commun. 2020, 11 (1).
(12) Kenkel, S.; Bhargava, R. Nanoscale Imaging of Biological Samples with Responsivity Corrected Atomic Force Microscopy-Infrared (AFM-IR) Spectroscopy. 2019, No. May, 51.

Rohit Bhargava
is Founder Professor of Engineering and serves as the Director of the Cancer Center at Illinois of the University of Illinois at UrbanaChampaign. His primary appointment is in the Department of Bioengineering with joint appointments in several engineering departments and Chemistry as well as in the Carle-Illinois College of Medicine. Rohit graduated with a dual-degree B.Tech. (1996) from the Indian Institute of Technology, New Delhi and received a doctoral degree from Case Western Reserve University (2000). After a stint at the National Institutes of Health, he has been at Illinois as Assistant (2005-2011), Associate (2011-2012) and Full (2012-) Professor. Rohit is widely recognized for his research on chemical imaging and advances in theory, instrumentation, and applications in cancer pathology. Current work in chemical imaging in his laboratory focuses on theoretical modeling that can push the limits of speed and quality of infrared spectroscopic imaging as well as its application in several novel areas. In particular, Rohit’s group aims to recognize and subtype cancer by its underlying molecular characteristics, by advanced chemical imaging and application of modern machine learning, ultimately allowing for better treatment of patients. His innovative teaching and mentoring has been consistently recognized by the success of his students. He conceived of and currently directs of the Tissue Microenvironment training program supported by a T32 grant from the NIH. Rohit has also served to connect the research community in new and exciting ways to take on basic science and engineering questions that surround cancer. He was the first assistant professor hired into Illinois’ Bioengineering Department and played a key role in its development. He proposed and has served for ~10 years to develop the Cancer Center at Illinois - a basic science center at the convergence of high quality technology and engineering and oncology.

Seth Kenkel
is a Postdoctoral Research Associate in the Chemical Imaging and Structures Laboratory at the University of Illinois at UrbanaChampaign. Seth earned his B.S. (2010) and M.S. (2012) degrees in Mechanical Engineering from the University of Illinois at UrbanaChampaign. After working for John Deere Dubuque Works (2012-2014), he received a doctoral degree in Mechanical Engineering (2014-2020) from the University of Illinois at Urbana-Champaign and was a Tissue Microenvironment fellow (2016-2018). His work focuses on the development of next-generation infrared spectroscopic imaging techniques guided by theoretical modeling from first principles to enable new chemical imaging capabilities at the micro and nanoscale. In particular, he has developed novel hardware and controls to improve limitations on accuracy and noise in nanoscale chemical imaging techniques enabling numerous applications in materials and life sciences. He received the EAS graduate student award (2019) in recognition of this work. His first principles design approach drives current progress in the development of novel instruments and algorithms to improve the quality of infrared photothermal imaging for wide adoption by novice users in many scientific disciplines.

Kevin Yeh
is Postdoctoral Research Associate at the Beckman Institute for Advanced Science and Technology of the University of Illinois at UrbanaChampaign. Kevin graduated with a bachelor’s degree (2009) in Biomedical Engineering from Johns Hopkins University, with a master’s degree (2011) in Biomedical Engineering from Cornell University, and received a doctoral degree from the University of Illinois at Urbana-Champaign (2019) in Bioengineering. His work in the Chemical Imaging and Structures Laboratory of Professor Rohit Bhargava focuses on the research and development of laser-based discrete frequency infrared microscopes for spectroscopic microscale chemical imaging. He has led the development efforts for multiple generations of custom-built imaging systems, each time pushing the performance of the instrumentation and demonstrating new benchmarks for resolution, noise, and speed. His current work aims to translate these advances into real-world platforms, accelerating the adoption of state-ofthe-art chemical imaging microscopes integrated with modern machine learning, thereby enabling better patient diagnostics in clinical environments as well as driving new research interests in material science, chemistry, and biology.