Polymer based Semiconductors
- 07 Jan 2019
- Volume 15
- NanoScientific Magazine, Winter 2019
Dr. Alberto Salleo
Associate Professor Materials Science & Engineering, Stanford University
Professor Salleo received his Laurea degree in Chemistry from the University of Rome and his M.S. and Ph.D. in Materials Science from UC Berkeley investigating optical breakdown in fused silica. He spent 5 years at the Palo Alto Research Center as a postdoc and then a member of the research staff in the Electronic Materials Laboratory before joining the Department of Materials Science and Engineering at Stanford University in 2005. Dr. Salleo has been a Principal Editor of MRS Communications since 2011.
While at Stanford, Salleo won the NSF Career Award, the 3M Untenured Faculty Award, the SPIE Early Career Award and the Tau Beta Pi Excellence in Undergraduate Teaching Award and the Gores Award for Excellence in Teaching, Stanford’s highest teaching honor. He has been a Thomson Reuters Highly Cited Researcher in Materials Science (top 1% cited scientists) since 2015.
The Salleo Research Group is interested in novel materials and processing techniques for large-area and flexible electronic/photonic devices. They also study defects and structure/property relations of polymeric semiconductors, as well as nano-structured and amorphous materials in thin films.
SCANNING TRANSMISSION ELECTRON MICROSCOPY
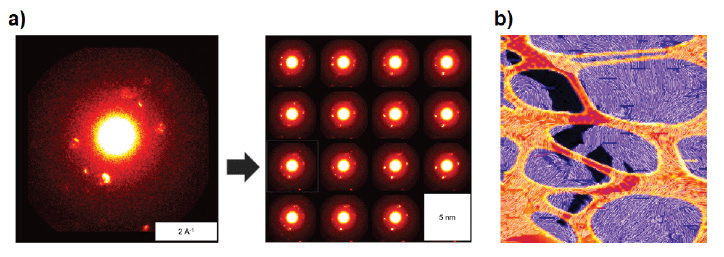
Scanning Transmission Electron Microscopy (STEM) gives spatially resolved electron diff raction images. Dr. Salleo’s group uses this technique to study how crystallite quality and orientation vary across a polymer film, and what implications this has for processing and performance.
(a) Diff raction spots indicate the local direction of polymer chains (left ), Comparing adjacent scans sheds light on microstructure (right). (b) Conjugated polymer (PBTTT, purple) on a lacy carbon support (orange). Lines show the orientation of polymer aggregates, calculated from STEM diff raction patterns.
Polymer-based Semiconductors and Neuromorphic Devices
Organic electronic materials off er an attractive option for polymer based semiconductor systems and could provide biocompatible and relatively inexpensive neuromorphic devices with low-energy switching and excellent tenability paving the way for neuromorphic computing to address the inherent limitations of conventional silicon technology in dedicated machine learning applications.
As Moore’s scaling law reaches an end, new brain-like (neuromorphic) computing architectures that embed memory and computation into a single device are highly sought aft er. Traditional semiconductor memory technology has yet to satisfy the needs of the “artificial synapse” that is the core of the neuromorphic computing architecture. This group uses organic semiconductors to mimic synaptic behavior in an electrochemical organic neuromorphic device (ENODe), which couples ionic and electronic currents to emulate the strength of neuronto-neuron connections. The high linearity and low switching energy of ENODes make them highly suitable for massively parallel neural algorithm accelerators, i.e. brain-like computer chips. Salleo’s research group focuses on leveraging the ionic/electronic transport properties of polymeric semiconductors to design novel devices for neuromorphic computing.
“In the electrical field there are ions and electrons moving in a field. The motion of the ions is transduced as sensors, thereby being used as electrochemical sensors. In the case of wearable sensors such as a “sweat sensor”, organic material which is able to carry both ions and electrons is used and a blend is used which carries ions. New polymers are always being tested as well as material that can carry ions, electrons or both. The advantage of organic polymers is the low cost and flexibility,” explains Dr. Salleo.
Q & A WITH DR. ALBERTO SALLEO,
Associate Professor StanfordDepartment of Materials Science and Engineering, Geballe Laboratory for Advanced Materials; Principal Editor MRS Communications
What imaging tools such as AFM do you use to study defects and properties/structures and is accurate imaging at nanoscale crucial in your research?
We typically use standard tapping mode AFM and look at both topography and phase. Accurate imaging at the nanoscale is crucial as we oft en work with materials that have very small microstructural features (e.g. polymer crystallites, phase separation), which control the functional properties of the materials.
Can you give a few examples of how you use AFM in your research?
We have used conductive tip AFM to study the distribution of dopants in doped conjugated polymers. We have also used tapping mode AFM to study the terracing of crystalline organic semiconductors and the topography of grain-boundaries.
What capabilities in AFM are most significant to assist you in research?
For general use, measuring roughness is important for us. Lately we have beeninterested in other capabilities such as the ability to measure current, electrochemical AFM and the possibility of using AFM in water.
In the future, what advances in AFM and nanometrology can you envision that would significantly improve the functionality?
Multimodal capabilities (i.e. measuring several properties simultaneously) would improve the study of functional materials as we oft en look for correlations between diff erent materials features.
What are the next significant advances you see in material science research and how will they impact society?
I think the integration of materials with living matter is poised to have an enormous impact in healthcare and possibly even computation.
Alberto Salleo, associate professor of materials science and engineering, withgraduate student Scott Keene characterizing the electrochemical properties of an artificial synapse for neural network computing. They are part of a team that has created the new device. (Image credit: L.A. Cicero)
STANFORD RESEARCHERS, LED BY PROFESSOR SALLEO CREATE A HIGH-PERFORMANCE, LOW-ENERGY ARTIFICIAL SYNAPSE FOR NEURAL NETWORK COMPUTING
Researchers at Stanford University and Sandia National Laboratories have made a progress that could help computers mimic one piece of the brain’s eff icient design – an artificial version of the space over which neurons communicate, called a synapse.
“It works like a real synapse but it’s an organic electronic device that can be engineered,” said Alberto Salleo, associate professor of materials science and engineering at Stanford. “It’s an entirely new family of devices because this type of architecture has not been shown before. For many key metrics, it also performs better than anything that’s been done before with inorganics.”
The new artificial synapse mimics the way synapses in the brain learn through the signals that cross them producing a significant energy savings over traditional computing because the processing creates the memory.This synapse may one day be part of a more brain-like computer, which could be especially beneficial for computing that works with visual and auditory signals. Examples of this are seen in voice-controlled interfaces and driverless cars. “In looking at neuromorphic computing, companies design neuro electrochemical devices
designed to emulate a synapse and neurotransmitters diff use their device. The strength of the connection can be molulated like a synapse, making a network similar to the brain. As we approach the end of Moore's Law in semiconductor like Carver Mead thought of 30 years ago, the advantage of neuromorphic computing is the very low power it requires, like a brain. Where I see this going is specialized chips instead of general purpose chips. For instance there can be a specific chip for computer vision. The silicon cmos chip is so powerful; I see neuromorphic chips as a compliment to them to augment this technology, not to replace it,” explains Dr. Alberto Salleo.
ORGANIC ELECTROCHEMICAL TRANSISTORS AND WEARABLE ELECTRONICS
Organic electrochemical transistors (OECTs) are of high interest due to their ability to transduce ionic signals, such as those produced in biology, with relatively high gain at low operating voltages (<0.5V), making them ideal candidates for biosensing applications. Our group’s research spans from elucidating fundamental structure-property relations in mixed ionic-electronic organic materials to developing novel wearable biosensors. We use characterization (i.e. synchrotron X-ray scattering, device modeling, spectroscopy, electrochemical measurements) to develop a fundamental understanding of organic materials for OECT devices. Extrapolating from fundamental principles, our group has developed various biomimetic membranes to selectively sense metabolites, ions and hormones from various bodily fluids including sweat and saliva.
In an article recently published in Science Advances (July 20, 2018), authors Onur Parlak, Scott Tom Keene, Andrew Marais, Vincenzo F. Curto and Alberto Salleo demonstrated the integration of an artificial receptor as a biomimetic polymeric membrane for stable and selective molecular recognition using OECTs to produce a wearable sweat diagnostics platform for real-time analysis of the human stress hormone cortisol. The group led by materials scientist Alberto Salleo at Stanford University has created a stretchy patch that applied directly to the skin, wicks up sweat and assesses how much cortisol a person is producing.
This wearable sensing device for cortisol detection was realized using a conductive polymer channel functionalized with a cortisol-selective membrane produced on a flexible and stretchable elastomeric substrate. The molecularly selective polymer-based membrane shows high chemical and physical stability at body temperature, as well as resistance to physical deformation. The presented sensor tolerates mechanical testing such as bending and stretching in conditions similar to those found in the normal range of motion of the human epidermis. Moreover, we used a simple strategy to generate a passive fluid control system consisting of a laser-patterned micro capillary channel array that provides fast and precise delivery of sweat directly to the sensor interface. The resulting wearable sensor was used for measuring cortisol concentration in a real human sweat sample collected during exercise. Considering that traditional blood analysis is often used for cortisol sensing, the wearable device provides many advantages including noninvasiveness, ease of operation, and user comfort. "We are particularly interested in sweat sensing, because it offers noninvasive and continuous monitoring of various biomarkers for a range of physiological conditions," said Onur Parlak, a postdoctoralscholar in the Salleo lab and lead author of the paper. "This offers a novel approach for the early detection of various diseases and evaluation of sports performance."
“Wearable electrochemical sensor can analyze sweat which occurs under stress.
The advantage of organic material is that it is soft and pliable, making it suitable for
wearing. These sensors can detect heart beat, blood pressure, blood oxidation and create bio feedback mechanisms for precision medicine. Research being done now is testing wearable electrochemical sensors worn in socks and printed on textiles so they are transparent to the user by being printed on textiles with organic integration. Evaluation of common household items such as a toilet and a toothbrush to analyze bodily secretions could be the health detection devices of the future,” explains Dr. Alberto Salleo.
The Stanford team is currently working to miniaturize the device, and also hoping to develop a user interface that will assist in the evaluation of data. The team also wants to adapt the device so that it could be powered by harvested energy rather than by an internal battery. The device could be modified to detect other hormones and non-charged ions within sweat. The end goal is to have a device that is capable of monitoring several different biomarkers at the same time, which would help researchers and health professionals gain a more holistic, individualized representation of a person’s health and mental state.
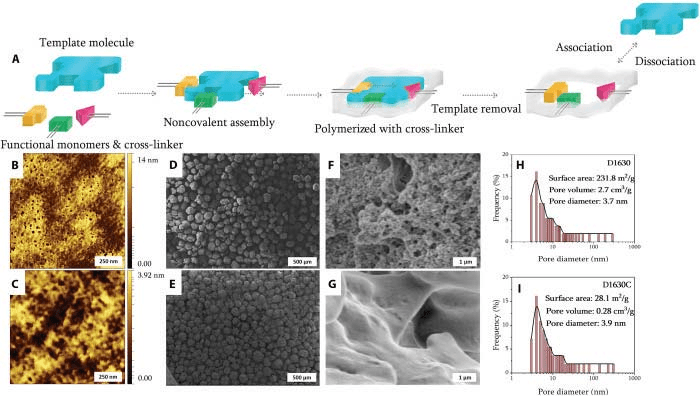
A) Molecular memory is introduced on the sensor surface by copolymerizing functional monomer and cross-linker in the presence of the analyte, which acts as a molecular template. After elution of the analyte, complementary binding sites are revealed complimentary in size and shape to template by creating molecular memory on the surface that allows specific rebinding of the target molecule. The recognition sites obtained in this manner have binding affinities approaching those demonstrated by antibody-antigen systems. Tapping-mode atomic force microscopy (AFM) analysis of cortisol-selective polymer (B) and its corresponding control (C). Scanning electron microscopy (SEM) images of cortisol-selectivepolymer (D and F) and its control (E and G) with two different magnifications. Pore size distribution for cortisol-imprinted (H) and nonimprinted polymers (NIPs) (I). The BJH method was applied to calculate pore size distribution from experimental isotherms using the Kelvin model of pore filling. The method applies only to the mesopore and small macropore size range.