Simultaneous Topographical and Electrochemical Mapping using Scanning Ion Conductance Microscopy
- 20 Aug 2018
- Volume 14
- NanoScientific Magazine, FALL 2018
Wenqing Shi, Gerald Pascual, Byong Kim, Keibock Lee Park Systems Inc., Santa Clara, CA USA
INTRODUCTION
Since the inception of scanning tunneling microscopy (STM) [1], electrochemists have sought to take advantage of scanned probe microscopy (SPM) techniques to manipulate the spatial position of a probe with high resolution to facilitate simultaneous high resolution topographical, conductometric, and amperometric/voltammetric imaging of surface and interfaces [2]. Lately, scanning ion conductance microscopy (SICM) [3], has emerged as a versatile non-contact imaging tool and been employed for a variety of applications. SICM has been used to investigate the surface topography of both synthetic and biological membranes [4, 5], ion transport through porous materials, dynamic properties of living cells [6, 7, 8], and suspended artificial black lipid membranes [9]. In addition, integration of complementary techniques with SICM has led to many exciting new applications, including scanning near-field optical microscopy (SNOM) [10] and patch-clamping [11, 12]. Powerful as it is, SICM remains insensitive to electrochemical properties, or, in other words, SICM is inherently chemically-blind and has no chemical specificity. To obtain spatially-resolved electrochemical information, scanning electrochemical microscopy (SECM), also known as the chemical microscope, has been developed. SECM has been widely employed to examine localized electrochemical properties and reactivity of various materials/interfaces, such as electrode surfaces and interfaces [13, 14, 15], membranes [16, 17, 18], and biological systems [19, 20, 21, 22, 23]. Despite its many applications, SECM, however, lacks reliable probe-sample distance control, and the probe is usually kept at a constant height during conventional SECM scanning. As a result, any variation in surface topography will result in changes in probe-sample distance, and thus leading to convolution to the measured faradaic current, which will complicate the subsequent data interpretation [18].
To address the above-mentioned issues for SICM and SECM, hybrid SICM-SECM techniques have been developed, in which the SICM compartment provides the accurate probe-sample distance control, while the SECM compartment measures the faradaic current for electrochemical information collection. Here in this application note, first, the principle of operation for SICM, SECM and SICM-SECM will be briefly discussed. Next, the probe as well as the sample that are used for SICM-SECM imaging experiments are described. Finally, simultaneous SICM-SECM topography imaging and electrochemical mapping with SmartScan RTM10e using a Park NX10 system is demonstrated.
Principle of Operation
Scanning Ion Conductance Microscopy (SICM)
In SICM operation, an electrolyte-filled nanopipette is used as the probe to raster scan an underlying substrate immersed in bath electrolyte (i.e., PBS buffer). A Ag/AgCl electrode is back-inserted in the pipette to serve as the working electrode, while another Ag/AgCl electrode is placed in the bath solution and used as the reference electrode. As a potential is applied between the two electrodes, an ion current is generated. The amplitude of the ion current is probe-substrate distance (Dp-s) dependent. The ion current decreases as the probe approaches the sample surface. This is a result of the ion flow being hindered between the probe tip and the underlying surface. This current-distance relationship can be obtained experimentally with an approach curve, where the ion current is plotted as a function of Dp-s . At every recorded Dp-s , a specific ion current is measured. This distance-dependent ion current is then used as a feedback to control probe position during scanning. As a result, the topographical map of the substrate under study is generated. However, the major cavity in the capability of SICM is its lack of chemical specificity.
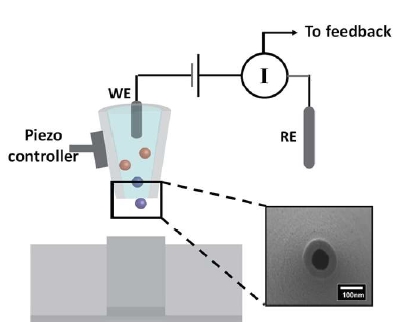
Figure 1. Fig. 1 SICM schematic illustration. The scanning probe consisted of a working electrode (WE, Ag/AgCl) back-inserted into a nanopipette. The reference electrode (RE) is placed in the bath solution (i.e., PBS buffer). The nanopipette opening is shown in the zoomed-in scanning electron micrograph. As a potential is applied between the WE and RE, a distance-dependent ion current is generated, based on which a piezoelectric positioner is used to maintain a constant probe-substrate distance during scanning. As a result, topographical information of the sample under study can be obtained.
Scanning Electrochemical Microscopy (SECM)
Scanning electrochemical microscopy employs a micro- or nanoelectrode, termed as the tip, to scan in the vicinity of a substrate. The bath solution consists of supporting electrolytes and an electrochemically active species. During a SECM experiment, the tip is biased at a sufficient potential such that the electrochemical active species will either be oxidized or reduced at the tip. By measuring the faradaic current, quantitative electrochemical information of the interfacial region will be obtained. Several imaging schemes have been developed for SECM operation, including negative feedback mode, positive feedback mode, and substrate generation/tip collection (SG/TC) mode. Feedback mode exploits the variation in the faradaic current as the tip approaches the substrate, with the sign (i.e., +/-) of the variation depending upon the conductivity of the surface. When the probe approaches a conductive surface, redox cycling will lead to an increase in the measured faradaic current, this is known as the positive feedback. Whereas if the probe moves close to an insulative surface, the diffusion of the redox molecules to the electrode surface will be hindered, and, thus, a decrease in faradaic current will be detected, also known as the negative feedback. In SG/TC mode, the sample and the tip are biased at different potentials, where the sample is biased to generate (oxidize or reduce) redox species in solution and the tip is biased to collect (reduce or oxidize) the species generated at the substrate.
Taken in total, the drawback in SECM lies in the fact that feedback-controlled probe positioning is complicated by the different faradaic current behavior over conductive and insulative substrates. In conventional SECM measurements, constant-height imaging is used, in which the tip is maintained at a constant height from the surface and the faradaic current is recorded. In constant-height SECM imaging, when variations in surface topography and reactivity occurs concurrently, the data interpretation becomes problematic.
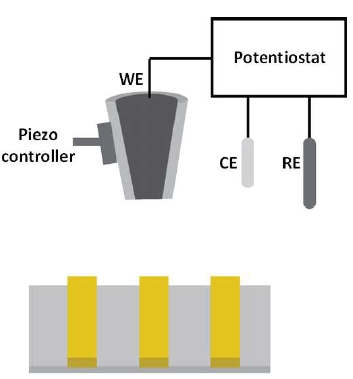
Figure 2. SECM schematic illustration. The electrochemical cell consisted of a working electrode (WE), a counter electrode (CE) and a reference electrode (RE). The bath solution contains supporting electrolyte and an electrochemically active species. During SECM operation, the WE is biased at a sufficient potential at which the redox reaction of the electrochemically active species occurs. By scanning the probe at a constant-height away from the underlying substrate while recording the faradaic current response, the electrochemical activity of the surface can be obtained.
Hybrid SICM-SECM
To overcome the limitations in constant-height SECM imaging, an alternative approach, SICM-SECM, has been developed. A number of pipette-based probes have been designed for SICM-SECM imaging. Bard and his colleagues have developed a gold-coated micropipette insulated with electrophoretic paint. [24] A similar approach has been reported by Hersam and his colleagues, in which the Au-coated pipette was first completed insulated by atomic layer deposition of Al2O3, followed by focus ion beam (FIB) milling to expose both the nanopore and gold electrode. [25] In an alternative approach, a theta pipette is used, with one barrel filled with electrolyte for SICM and the other barrel filled with pyrolyzed carbon for SECM. [26] In SICM-SECM operation, the probe-sample distance can be controlled via the SICM compartment and the electrochemical activity can be measured via the SECM compartment, enabling truly independent concurrent topographical and electrochemical imaging.
Experimental
Sample
As shown in Figure 3, the standard sample used for SICM-SECM experiment described herein consists of Au bars that are 10 μm in width and 300 nm in height on Pyrex substrate. The pitch width is 20 μm.
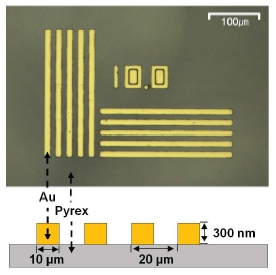
Figure 3.SICM-SECM standard sample. The sample consisted of Au barswith a width of 10 μm and a height of 300 nm on Pyrex substrate. Thepitch width is 20 μm.
Probe
The probe used herein is adopted from a method described in Shi et al. [18]. In brief, nanopipettes obtained via laser pulling were first coated with 10 nm Cr adhesion layer, followed by 200 nm Au layer by thermal evaporation. Next, chemical vapor deposition of parylene C was performed such that the Au-coated nanopipettes were completely covered by parylene C. Finally, a focused ion beam (FIB) technique was used to expose the nanopore and the Au crescent. A representative scanning electron micrograph of the SICM-SECM probe is shown in Figure 4. The diameter of the central pore ranges from 200 – 250 nm. The Au crescent thickness is ~200 nm.
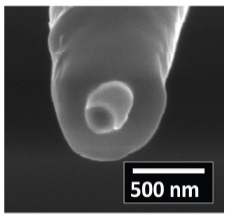
Figure 4. Representative scanning electron micrograph of the SICM-SECM probe. Pore opening ~250 nm. Au crescent coating ~200 nm.
Cyclic Voltammetry
Prior to SICM-SECM imaging, cyclic voltammetry (CV) in a bulk solution consisted of 100 mM KCl and 10 mM Ru(NH3)63+ was performed. The purpose of the CV measurement is to 1) characterize the Au crescent electrode performance and 2) to choose the potential at which the Au crescent electrode will be biased at during SICM-SECM imaging experiments.
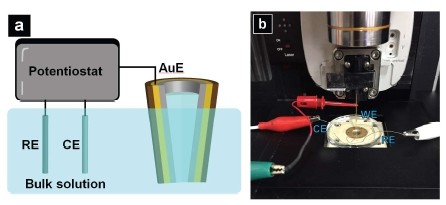
To carry out the CV measurement, a potentiostat (Model 760E, CH instrument, Austin, TX) with a three-electrode electrochemical cell is used. The Au crescent electrode served as the working electrode (WE) with respect to a Ag/AgCl reference electrode (RE) and a Pt counter electrode (CE) in the bulk solution, as indicated in the cartoon illustration seen in Figure 5a. In Figure 5b, setup of the CV measurement performed with the probe mounted on the SICM head of a Park NX10 system is shown. The potential window used here is from 0 to -0.5 V, with a step size of 0.1 V/s.
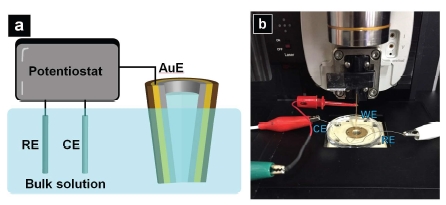
Figure 5.a) Cartoon illustration of CV measurement; b) Setup of the CV measurement performed with the probe mounted on the SICM head of a Park NX10 system.
SICM-SECM Imaging
To realize SICM-SECM imaging, a Park NX10 system in combination with an ammeter (Chem Clamp, Dagan Corporation, Minneapolis, MN) is used. A schematic diagram of the SICM-SECM setup is depicted in Figure 6a. Ion current between the Ag/AgCl electrodes inside the pipette (PE: pipette electrode) and another Ag/AgCl pallet electrode in the bath solution (RE: reference electrode) is employed as feedback to control probe-substrate distance. The Au crescent electrode (AuE) is used to acquire electrochemical signal. The potential applied between the PE and RE was 0.1 V, and the z To realize SICM-SECM imaging, a Park NX10 system in combination with an ammeter (Chem Clamp, Dagan Corporation, Minneapolis, MN) is used. A schematic diagram of the SICM-SECM setup is depicted in Figure 6a. Ion current between the Ag/AgCl electrodes inside the pipette (PE: pipette electrode) and another Ag/AgCl pallet electrode in the bath solution (RE: reference electrode) is employed as feedback to control probe-substrate distance. The Au crescent electrode (AuE) is used to acquire electrochemical signal. The potential applied between the PE and RE was 0.1 V, and the potential at the Au E was held at -0.5 V. The ammeter is used for both applying potential to the AuE and measuring the faradaic current at the AuE. The measured faradaic current is then fed to one of the auxiliary recording channels (AUX IN 2) of the NX10 controller and recorded in real-time via the SmartScan software. As a result, simultaneous topographical imaging (from SICM) and electrochemical activity mapping (from SECM) is accomplished.
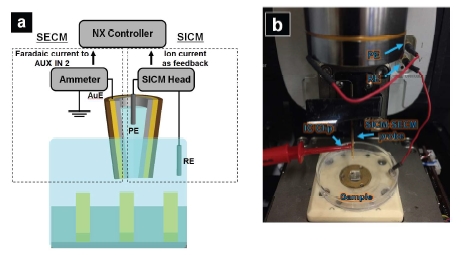
Figure 6. a) Cartoon illustration of CV measurement; b) Setup of the CV measurement performed with the probe mounted on the SICM head of a Park NX10 system.
Results and Discussion
As shown in Figure 7, five consecutive CVs taken with the Au crescent electrode in a bulk solution containing 100 mM KCl + 10 mM Ru(NH3)63+ are shown. For Ru(NH3)63+ , starting from ~0.25 V, the reduction reaction of Ru(NH3)63+ to Ru(NH3)62+ takes places, and as the potential is ramped up, at ~-0.5 V, the electrochemical reaction is diffusion-controlled and a steady-state current is reached. The electrochemical reaction is shown below:
![]()
From the CV data, a steady-state current was reached at -0.5 V, and, thus, in the following SICM-SECM imaging experiment, the bias applied to the Au electrode will be kept at -0.5 V.
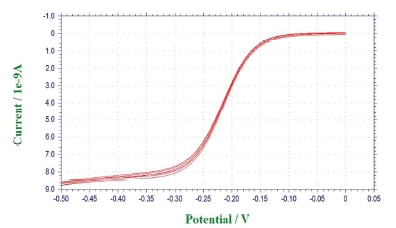
Figure 7.Cyclic voltammograms (CVs) taken with the Au crescent electrode in bulk solution containing 100 mM KCl and 5 mM Ru(NH3)63+.
In Figure 8, representative SICM-SECM images are shown. In Figure 8a, topography image of the Au/Pyrex pattern is shown. Figure 8c (top) shows the line profile of the topography image. The measured pitch width is 20.06 μm, which matches the actual pitch width (20 μm). The measured height of the Au bar is 302.03 nm, which is, again, agrees with the actual feature height of 300 nm. In Figure 8b, electrochemical activity map of the same region seen in Figure 8a is shown. The absolute value of the faradaic current over Au is ~4.5 nA, while over Pyrex, the absolute value of the faradaic current is ~3.6 nA. An overall ~981 pA faradaic current difference is observed. Correlation of the topography and faradaic current images reveals the expected contrast, with enhanced faradaic current over the conductive Au regions, consistent with positive feedback due to redox cycling, and reduced Faradaic current over insulative Pyrex trenches, consistent with negative feedback due to hindered diffusion.
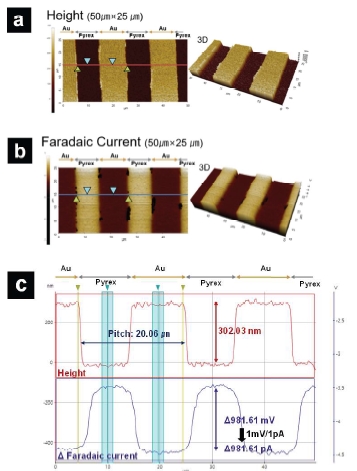
Figure 8. Representative SICM-SECM images. a) SICM topography image; b)SECM faradaic current image. c) Line profile along the line seen in a) and b). Image size: 50 μm × 25 μm.
CONCLUSIONS
In this application note, we demonstrated the use of a Park NX10 AFM in combination with an ammeter for concurrent topography imaging and electrochemical mapping. The SICM-SECM probe utilized here consisted of a Au crescent electrode (AuE) on the peripheral of a nanopipette. High resolution probe-substrate distance control was obtained by the ion current feedback from SICM, while simultaneous electrochemical signal collection was achieved via the AuE from SECM. As a proof-of-concept experiment, a Au/Pyrex pattern standard sample was imaged with the SICM-SECM technique. The Au bar and the Pyrex substrate were clearly resolved from the SICM topography image, with the bar height and pitch width closely matching the actual values. In terms of the electrochemical property mapping, higher faradaic current was seen when the probe was scanned over Au bar as a result of redox cycling, while lower faradaic current was observed when the probe was over Pyrex substrate due to hindered diffusion. The capability of the SICM-SECM technique described here holds promise of many exciting applications in the field of electrochemistry, material science and battery research.
References
[1] Binnig, G., Rohrer, H., Gerber, C. and Weibel, E., Tunneling through a controllable
vacuum gap. Appl. Phys. Lett., 1982, 40, 178-180.
[2] Snowden, M. E., Güell, A. G., Lai, S. C., McKelvey, K., Ebejer, N., O’Connell, M. A.,
Colburn, A. W. and Unwin, P. R. Scanning electrochemical cell microscopy: Theory
and experiment for quantitative high resolution spatially-resolved voltammetry and
simultaneous ion-conductance measurements. Anal. Chem., 2012, 84, 2483-2491.
[3] Hansma, P. K., Drake, B., Marti, O., Gould, S. A. C. and Prater, C. B. The scanning
ion-conductance microscope. Science, 1989, 243, 641.
[4] Chen, C. C., Derylo, M. A. and Baker, L. A. Measurement of ion currents through
porous membranes with scanning ion conductance microscopy. Anal. Chem., 2009,
81, 4742-4751.
[5] Korchev, Y. E., Bashford, C. L., Milovanovic, M., Vodyanoy, I. and Lab, M. J.
Scanning ion conductance microscopy of living cells. Biophys. J., 1997, 73, 653-658.
[6] Shevchuk, A. I., Gorelik, J., Harding, S. E., Lab, M. J., Klenerman, D. and Korchev,
Y. E. Simultaneous measurement of Ca 2+ and cellular dynamics: combined scanning
ion conductance and optical microscopy to study contracting cardiac myocytes.
Biophys. J., 2001, 81, 1759-1764.
[7] Gorelik, J., Shevchuk, A. I., Frolenkov, G. I., Diakonov, I. A., Kros, C. J.,
Richardson, G. P., Edwards, C.R., Klenerman, D. and Korchev, Y. E. Dynamic assembly
of surface structures in living cells. Proc. Natl. Acad. Sci. U.S.A., 2003, 100,
5819-5822.
[8] Pellegrino, M., Orsini, P. and De Gregorio, F. Use of scanning ion conductance
microscopy to guide and redirect neuronal growth cones. Neurosci. Res., 2009, 64,
290-296.
[9] Böcker, M., Muschter, S., Schmitt, E. K., Steinem, C. and Schäffer, T. E. Imaging
and patterning of pore-suspending membranes with scanning ion conductance
microscopy. Langmuir, 2009, 25, 3022-3028.
[10] Korchev, Y. E., Raval, M., Lab, M. J., Gorelik, J., Edwards, C. R., Rayment, T. and
Klenerman, D. Hybrid scanning ion conductance and scanning near-field optical
microscopy for the study of living cells. Biophys. J., 2000, 78, 2675-2679.
[11] Gorelik, J., Gu, Y., Spohr, H.A., Shevchuk, A.I., Lab, M.J., Harding, S.E., Edwards,
C.R., Whitaker, M., Moss, G.W., Benton, D.C. and Sánchez, D., Ion channels in small
cells and subcellular structures can be studied with a smart patch-clamp system.
Biophys. J., 2002, 83, 3296-3303.
[12] Shi, W., Zeng, Y., Zhou, L., Xiao, Y., Cummins, T.R. and Baker, L.A., Membrane
patches as ion channel probes for scanning ion conductance microscopy. Faraday
discussions, 2016, 193, 81-97.
[13] Holt, K.B., Bard, A.J., Show, Y. and Swain, G.M., Scanning electrochemical
microscopy and conductive probe atomic force microscopy studies of
hydrogen-terminated boron-doped diamond electrodes with different doping levels.
J. Phys. Chem. B, 2004, 108, 15117-15127.
[14] Shao, Y. and Mirkin, M.V., Probing ion transfer at the liquid/liquid interface by
scanning electrochemical microscopy (SECM). J. Phys. Chem. B, 1998, 102,
9915-9921.
[15] Yamada, H., Ogata, M. and Koike, T., Scanning Electrochemical Microscope
Observation of Defects in a Hexadecanethiol Monolayer on Gold with Shear
Force-Based Tip Substrate
Positioning. Langmuir, 2006, 22, 7923-7927.
[16] Uitto, O.D. and White, H.S., Scanning electrochemical microscopy of membrane
transport in the reverse imaging mode. Anal. Chem., 2001, 73, 533-539.
[17] Wilburn, J.P., Wright, D.W. and Cliffel, D.E., Imaging of voltage-gated
alamethicin pores in a reconstituted bilayer lipid membrane via scanning
electrochemical microscopy. Analyst, 2006, 131, 311-316.
</>[18] Shi, W. and Baker, L.A., Imaging heterogeneity and transport of degraded Nafion
membranes. RSC Adv., 2015, 5, 99284-99290.
[19] Bard, A.J., Li, X. and Zhan, W., Chemically imaging living cells by scanning
electrochemical microscopy. Biosens. Bioelectron., 2006, 22, 461-472.
[20] Bauermann, L.P., Schuhmann, W. and Schulte, A., An advanced biological
scanning electrochemical microscope (Bio-SECM) for studying individual living cells.
Phys. Chem. Chem. Phys., 2004, 6, 4003-4008.
[21] Adams, K.L., Puchades, M. and Ewing, A.G., In vitro electrochemistry of
biological systems. Annu. Rev. Anal. Chem., 2008, 1, 329-355.
[22] Amemiya, S., Guo, J., Xiong, H. and Gross, D.A., Biological applications of
scanning electrochemical microscopy: chemical imaging of single living cells and
beyond. Anal. Bioanal. Chem., 2006, 386, 458-471.
[23] Edwards, M.A., Martin, S., Whitworth, A.L., Macpherson, J.V. and Unwin, P.R.,
Scanning electrochemical microscopy: principles and applications to biophysical
systems. Physio. Meas., 2006, 27, R63.
[24] Walsh, D.A., Fernandez, J.L., Mauzeroll, J. and Bard, A.J., Scanning
electrochemical microscopy. 55. Fabrication and characterization of micropipet
probes. Anal. Chem., 2005, 77, 5182-5188.
[25] Comstock, D.J., Elam, J.W., Pellin, M.J. and Hersam, M.C., Integrated
ultramicroelectrodenanopipet
probe for concurrent scanning electrochemical
microscopy and scanning ion conductance microscopy. Anal. Chem., 2010, 82,
1270-1276.
[26] Takahashi, Y., Shevchuk, A.I., Novak, P., Zhang, Y., Ebejer, N., Macpherson, J.V.,
Unwin, P.R., Pollard, A.J., Roy, D., Clifford, C.A. and Shiku, H., Multifunctional
nanoprobes for nanoscale chemical imaging and localized chemical delivery at
surfaces and interfaces. Angew. Chem. Int. Ed., 2011, 50, 9638-9642.