Park AFM Scholarship Awards - Liliang Huang
- 21 Aug 2019
- Volume 17
- NanoScientific Magazine, Summer 2019

Liliang Huang is from The Mirkin Research Group at Northwestern University which has pioneered the use of nanoparticlebiomolecule conjugates as synthons in materials science and the development of many nanoparticle-based extra and intracellular biodiagnostic and therapeutic tools.
Research summary
Hydrogen is widely used as a clean, renewable alternative to fossil fuels, while water electrolysis represents an increasingly important method for industrial hydrogen manufacturing (~5% of the market). Currently, platinum is extensively used as a hydrogen evolution reaction (HER)electrocatalyst for water electrolysis; however, its scarcity and high cost limits practicality in electrolyzers. The key motivation of my graduate studies is focused on developing novel strategies to discover, design and fabricate costeffective HER electrocatalysts.
Nanoparticles are emerging as attractive HER electrocatalysts for researchers due to their enhanced activity when compared to their bulk counterparts. Pt-based multimetallic nanoparticles are particularly interesting because alloying Pt with earth abundant transition metal elements (e.g. Cu) is found to be favorable for activity and stability enhancement, in addition to the apparent cost savings. A series of multimetallic nanoparticle synthesis methods (e.g. coreduction and thermal decomposition) have been used to prepare targeted multimetallic nanocrystals with specific elemental compositions and size. These methods, however, are usually system-specific and lack in generalizability. Recently, the Mirkin group reported a novel synthesis method termed scanning probe block copolymer lithography (SPBCL), which allowed for the synthesis of a library of multimetallic nanoparticles of up to five elements (Au, Ag, Cu, Co, and Ni) by using a simple two-step thermal annealing treatment. This synthetic route forms a versatile platform for studying the catalysis-related properties of the nanoparticles. In this work, by effectively utilizing SPBCL in combination with density functional theory (DFT) calculations, we introduced a novel strategy to design and discover cost-effective HER electrocatalysts. Through this exercise, we have identified a homogeneous alloy PtAuCu nanoparticle as a more efficient and stable HER catalyst than the commercial Pt/C catalyst.
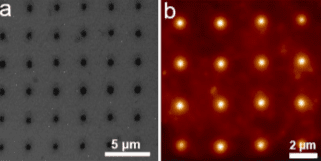
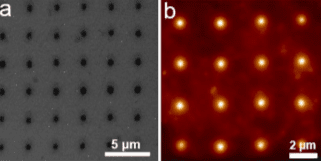
In an acidic electrolyte, the catalyst hydrogen binding energy (HBE) is the sole reaction descriptor for HER activity and an optimal HER catalyst should have an HBE that is neither too strong nor too weak. We first used density functional theory (DFT) calculations to evaluate the HBE for a series of multimetallic nanoparticles and identified PtAuNi and PtAuCu alloy nanoparticles as promising HER catalysts. Then SPBCL was used to accordingly synthesize these nanoparticles (Fig. 1), followed by characterization of their catalytic properties towards HER. Through this process, we confirmed PtAuCu alloy nanoparticles as excellent HER catalysts and highlighted the phase structure as a key parameter in determining the HER performance of the nanocatalysts, where the phase separation withinPtAuNi heterodimer particles (PtNi forms one phase domain and Au forms another domain) results in poor HER catalytic activities.
Finally, a bulk synthetic method was used to scale up the discovered PtAuCu catalyst, which was determined to be 7 times more active on a Pt-to-Pt basis than the “gold standard” commercial Pt catalyst. Herein, this work is a proof of-concept for a powerful tool used to discover new catalysts. We believe that more variables could be explored with this technology, leading to catalysts that will likely even exceed in performance than the one we identified here through preliminary efforts.
How do you use Park AFM in your research?
Park AFM instrument is quite important for my research. I not only use it as a multimetallic nanoparticle synthesis instrument, but also employ it to characterize the size and height of the patterned polymer nanoreactor features. Specifically, I synthesize nanoparticles through a process called SPBCL, where polymer pen arrays were placed into a modified AFM instrument and brought into contact with a flat substrate to pattern uniform polymer nanoreactors. These nanoreactors can further be thermally treated to produce nanoparticles. During the nanoparticle synthesis process, I will also use Park AFM to characterize the size uniformity of the patterned polymer features. Furthermore, the height and size ratio information of the patterned polymer features obtained from AFM measurements can also give me an idea of the hydrophobicity of the substrate, which is an important concern for this nanoparticle synthesis process.
Why do you think Park AFM is a valuable tool for researchers?
Nanotechnology has provided additional opportunities for researchers to address existing energy, environmental, and biological-related problems that are difficult to be solved with conventional bulk materials. Park AFM, as a flagship AFM instrument, has enabled researchers to obtain precise topographical information about the nanostructures, which is highly important for nanofabrication and its applications.